Projects
Differentiation of pluripotent stem cells into defined neuronal and glial subtypes
We have long-lasting experience in neural differentiation of pluripotent stem cells into neural progeny. We established a population of long-term self-renewing neuroepithelial-like stem (lt-NES) (Koch et al., 2009; Falk*, Koch* et al., 2012). These cells homogeneously express neural stem cell markers and, while maintaining a stable neurogenic and gliogenic differentiation potential, can be expanded across more than 100 passages. Remarkably, even after extensive passaging these cells can be efficiently recruited into different neuronal fates such as tyrosine hydroxylase-positive neurons or motorneurons.
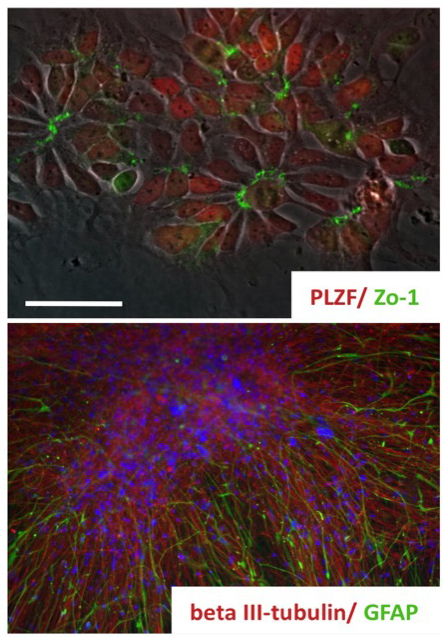
Koch, P., Opitz, T., Steinbeck, J., Ladewig, J., Brüstle, O. “A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration” Proc Natl Acad Sci U S A. Mar 3;106(9):3225-30. (2009) (IF: 9.971)
Falk, A.*, Koch, P.*, Kesavan, J., Takashima, Y., Ladewig, J., Alexander, M., Smith, A., Brüstle, O. “Capture of neuroepithelial-like stem cells from pluripotent stem cellsprovides a versatile system for in vitro production of human neurons” (* equal contribution) PLoS One 7(1):e29597. (2012) (IF: 4.411)
Stem Cell-Based Models for Neurodegenerative Disorders
Towards pathological protein processing and toxicity in Machado-Joseph-Disease (Spinocerebellar Ataxia Type 3 - SCA3)
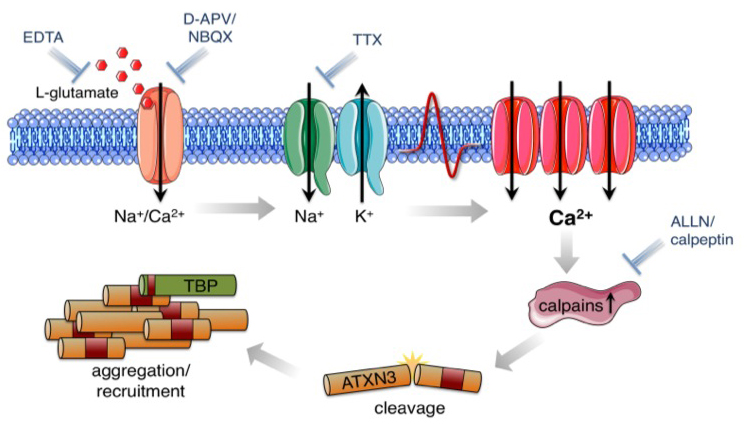
We identify early pathophysiological events in protein aggregation using reprogrammed disease-specific cells from patients with Machado-Joseph Disease (a polyglutamine disorder and the most common form of inherited ataxia). We could demonstrate that L-glutamate-induced excitation of MJD-neurons is sufficient to initiate cleavage of ATXN3 and the formation of SDS-insoluble aggregates, a hallmark of this disease. Aggregate formation was efficiently blocked by calpain inhibitors but not by caspase inhibition, indicating that excitation-induced activation of calpains is sufficient to generate aggregation-competent proteolytic polyQ fragments (Koch et al., 2011). We are currently using this model to study proteostasis, proteotoxicity and transcriptional dysregulation in MJD.
The project is funded by the E-RARE NET (joint transnational European Research Projects on Rare Diseases Driven by Young Investigators) and the National Ataxia Foundation (USA). (www.pppt-mjd.com)
Koch, P., Breuer, P., Peitz, M., Jungverdorben, J., Kesavan, J., Poppe, D., Doerr, J., Ladewig, J., Mertens, J., Tüting, T., Hoffmann, P., Klockgether, T., Evert, B.O., Wüllner, U., Brüstle, O. “Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease.” Nature. Nov 23;480(7378):543-6. (2011) (IF: 38.597)
Stem cell-based models for Alzheimer’s Disease
Using pluripotent stem cell-derived neurons we developed cellular models for Alzheimer’s Disease, the most prevalent neurodegenerative disorder in the western societies. We showed that the major proteins involved in Alzheimer’s Disease, including the amyloid precursor protein (APP), BACE1 and components of the γ-secretase complex are endogenously expressed in these cells. Neurons derived from pluripotent stem cells exhibited a maturation-dependent increase in Aβ secretion. Stable expression of the early onset familial AD-associated presenilin-1 mutation L166P resulted in a partial loss of γ-secretase function and an increased ratio of Aβ42/40 (Koch et al., 2012).
Overexpression of pseudo-hyperphosphorylated and wildtype MAPT (Tau) in PSC-derived neurons revealed that conformational changes of Tau could cause impairment of the axonal transport of mitochondria. When exposed to oxidative stress, misfolded Tau but not normal Tau led to progressive axonal disruption and neuronal degeneration. These studies suggest that normal Tau alone is insufficient to induce neuronal cell death while aberrant Tau species critically increase the vulnerability of authentic human neurons to oxidative stress leading to neurodegeneration (Mertens et al., 2013).
Using fibroblasts form inherited forms of Alzheimer’s Disease we have generated induced pluripotent stem cells and iPSC-derived neurons carrying mutations in the amyloid precursor protein APP and Presenilin-1. Analysis of secreted Aβ isoforms in these cells revealed that patient-derived cells have an elevated ratio of Aβ42/40, a disease causing pathomechanism in Alzheimer’s Disease. While γ-secretase modulators (GSMs), amongst them a set of non-steroidal anti-inflammatory drugs (NSAIDs), were found to lower Aβ42 in various model systems, NSAID-based GSMs surprisingly proved inefficient in human clinical trials. Reasoning that non-human and non-neuronal cells typically used in pharmaceutical compound validation might not adequately reflect drug responses of human neurons, we here used human pluripotent stem cell-derived neurons from AD patients and unaffected donors to explore the efficacy of NSAID-based γ-secretase modulation. We found that pharmaceutically relevant concentrations of these GSMs that are clearly efficacious in conventional non-neuronal cell models fail to elicit any effect on Aβ42/40 ratios in human neurons. Our work reveals a yet unrecognized resistance of human neurons to NSAID-based γ-secretase modulation, thereby stressing the necessity to validate compound efficacy directly in the human cell type affected by the respective disease (Mertens et al., 2013).
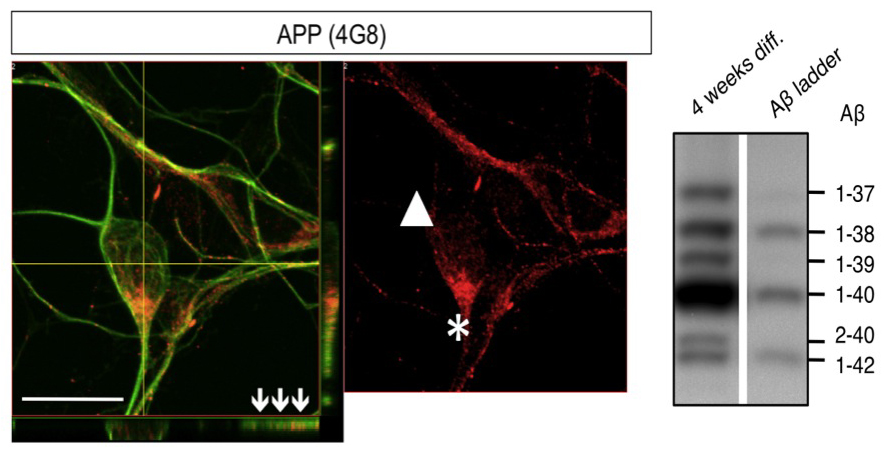
Mertens, J., Stüber, K., Wunderlich, P., Ladewig, J., Vandenberghe, R., Vandenbulcke, M., van Damme, P., Walter, J., Brüstle, O*., and Koch, P*., “APP Processing in Human Pluripotent Stem Cell-Derived Neurons is Resistant to NSAID-based γ-Secretase Modulation” Stem Cell Reports 1(6):491-8. doi: 10.1016/j.stemcr.2013.10.011. (2013) (IF: no IF yet)
Mertens, J., Stüber, K., Poppe, D., Doerr, J., Ladewig, J., Brüstle, O.*, Koch, P.*
„Embryonic stem cell-based modeling of tau pathology in human neurons“
Am J Pathol 182(5):1769-1779 (2013) (IF: 5.224)
Koch, P., Taboli, I., Mertens, J., Ladewig, J., Stüber, K., Brüstle., O., Walter, J. “Presenilin-1 L166P Mutant Human Pluripotent Stem Cell-Derived Neurons Exhibit Partial Loss of γ-Secretase Activity in Endogenous Amyloid-ß Generation” Am J Pathol. 180(6):2404-2416 (2012) (IF: 5.224)
Generation of human neurons by direct conversion of human fibroblasts
Applying latest direct programming technologies we developed culture conditions for the efficient direct conversion of human fibroblasts into functional neurons. Using only two transcription factors together with chemically defined culture conditions these protocol enabled direct conversion efficiencies of up to over 200% (2 neurons per fibroblast; Ladewig et al., 2012; Ladewig*, Koch* et al., 2013).
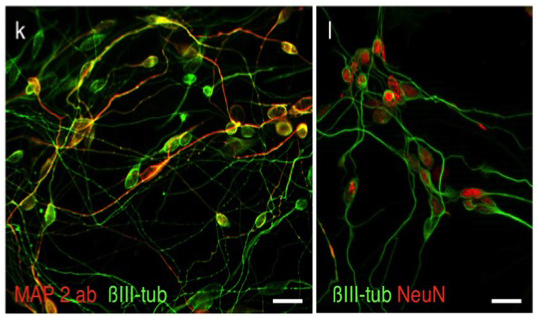
Ladewig, J., Mertens, J.*, Kesavan, J.*, Doerr, J., Poppe, D., Glaua, F., Herms, S., Wernet, P., Kögler, G., Müller, F.-J., Koch, P.*, Brüstle, O.* (2012) “Small molecules enable highly efficient neuronal conversion of human fibroblast” (* corresponding authors) Nature Methods 9(6):575-578 (2012) (IF: 23.565)
Ladewig, J.*, Koch, P.*, Brüstle, O. “Leveling Waddington: The Emergence of Direct Programming and the Loss of Cell Fate Hierarchies” Nature Reviews Molecular Cell Biology 14(4):225-236 (2013) (*equal contribution) (IF: 39,123)
